Technology Approach
WHAT ARE IMUNEXINSTM AND HOW ARE THEY APPLIED?
ImunexinsTM are engineered from a naturally occurring, non-antibody, human protein sequence with physical properties similar to those of the binding regions of antibody V-domains (imunexinsTM are referred to as V-like domains). ImunexinsTM have 3 binding loops akin to the binding loops of antibodies which can be engineered to bind to any target of interest. The unique physical properties of imunexinsTM allows attachment to other protein sequences including antibodies and enzymes (known as the parents) without perturbation of either the parent antibody or enzyme sequence or the imunexinTM and maintaining the parent's properties and manufacturability. ImunexinsTM are easily genetically fused to the parent protein via simple standard cloning protocols.
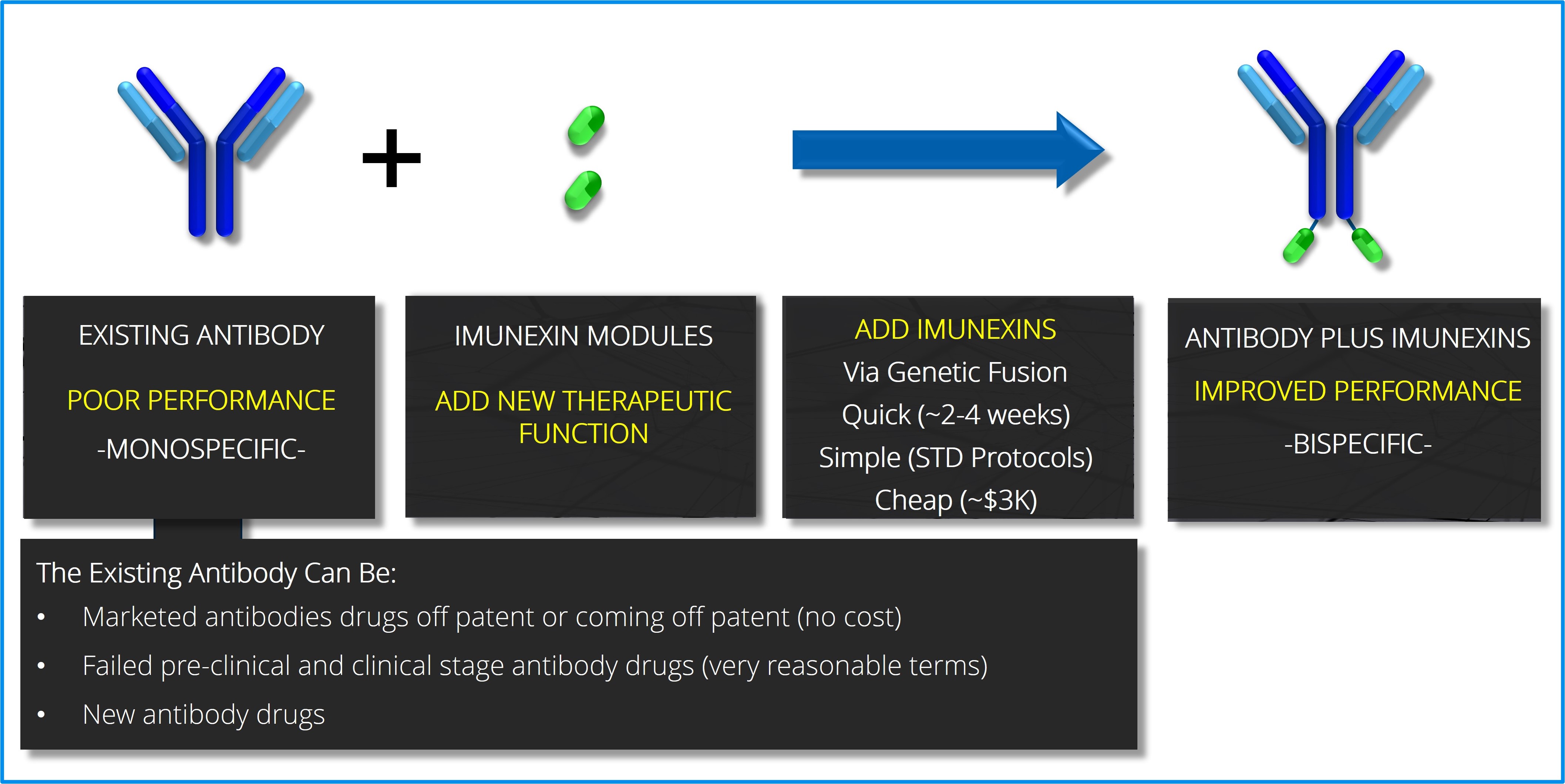
Diagram illustrating that imunexinsTM are added to existing antibodies via a simple genetic fusion (cloning event) into the expression vector of the antibody.

Diagram illustrating that two different imunexinsTM with different therapeutic properties are added to existing antibodies via simple genetic fusions (cloning events) into both the heavy and light chain expression vectors of the antibody.
WHAT ARE THE ADVANTAGES OF THE IMUNEXIN APPROACH?
Key advantages the ImunexinTM bispecific generation platform include:
Imunexin properties:
- ImunexinsTM are human in origin and are potentially less immunogenic.
- No reformatting or engineering is required post discovery stage.
- Can be genetically fused to any protein sequence without affecting the folding or expression of either the recipient protein or the imunexinTM.
- Discovery is via high throughput screening of our 10+10 randomized library via the Company’s proprietary HTP screening technology.
Simple and highly efficient bispecific antibody generation:
- Applicable to any antibody sequence.
- No antibody to imunexinTM matching needed.
- Antibody retains original binding sites and specificity.
- Proper pairing of antibody heavy and light chains.
- ImunexinsTM are simply cloned into exiting IgG vectors to generate a bispecific with no additional protein engineering required.
The parent antibody retains its original structure and function:
- The antibody’s original sequence, specificity and functions fully retained.
- Antibody retains its binding avidity.
- ImunexinsTM fused to antibodies also show binding avidity.
- Antibody-mediated effector functions are retained or can be modulated for select applications.
- Antibody pharmacokinetics including serum half-life are retained.
Simple and highly efficient bispecific enzyme generation:
- Applicable to any monomeric enzyme sequence.
- No enzyme to imunexinTM matching needed.
- Enzyme retains original active site and activity.
- ImunexinsTM are simply cloned into exiting protein vectors to generate an enzyme bispecific with no additional protein engineering required.
Integrates seamlessly into existing production processes:
- The manufacturability of the protein-imunexinTM bispecific mirrors those of the parent antibody or enzyme.
- Manufacturing, purification, formulation and all other processes are based on standard processes and remain the same as the parent protein.
GENERATING MULTISPECIFC MOLECULES
Antibodies: Imunexus converts parent antibodies from single binding antibodies into dual or tri binding antibodies by linking imunexinTM “modules” directly to the parent antibody. In essence, Imunexus takes well validated or commercial monoclonal antibodies that have one therapeutic binding region and converts them into bispecific or trispecific antibodies that have two or three therapeutic binding regions. One key advantage of this approach is that imunexinTM modules can be added to existing antibodies without modifying the sequence or any of the properties of the parent antibody while seamlessly adding additional therapeutic functionality to the original antibody.
One strength of Imunexus’ multispecific approach for generating therapeutics is predicated on the fact that there are an abundant number of parent antibodies available to Imunexus. Parent antibodies based on commercial antibodies that are approaching, or are already off patent, are readily available at no cost as well as numerous failed or poorly performing clinical and preclinical antibodies.
The imunexinTM approach to bispecific antibody drug development has a number of clear advantages:
- The fusion of imunexinsTM to antibodies is a simple process that does not appear to affect the physical or binding properties or manufacturability of either the parent antibody or the attached imunexinsTM. This gives Imunexus the potential to quickly develop unique drug candidates from almost any antibody, with flexibility and through-put that is not possible with most other bispecific antibody approaches.
- Imunexus believes that this approach has reduced clinical development risk as commercial, clinical or advanced preclinical parent antibodies have a strong scientific rationale supported by extensive preclinical or human clinical data and a clear roadmap to how these antibodies were developed.
- A clear advantage is speed, cost, and productivity. Unlike most other antibody therapeutic programs, once we identify and optimize an appropriate imunexin, we can fuse the same imunexinTM to any number of parent antibodies to quickly generate extensive pipelines. We can develop these bispecifics from concept to investigational new drug candidate comparatively quickly and for significantly less cost relative to traditional drug discovery approaches.

Diagram illustrating the potential for rapid pipeline expansion via the same imunexinTM modules added to a number of different existing antibody drugs to quickly create multiple potential products
Enzymes: Attaching imunexinsTM to enzymes converts the enzyme into a non-antibody bispecific. Like bispecific antibodies, the imunexinTM adds an additional therapeutic utility to the enzyme without impacting on the properties of the enzyme. As with antibodies, Imunexus takes well validated or commercial enzyme treatments and converts them into bispecifics with an additional property by fusing imunexinsTM to the enzyme. Again, there are an abundant number of parent enzymes available to Imunexus including commercial enzyme replacement therapies, that are approaching, or are already off patent, as well as numerous failed or poorly performing clinical and preclinical enzymes.
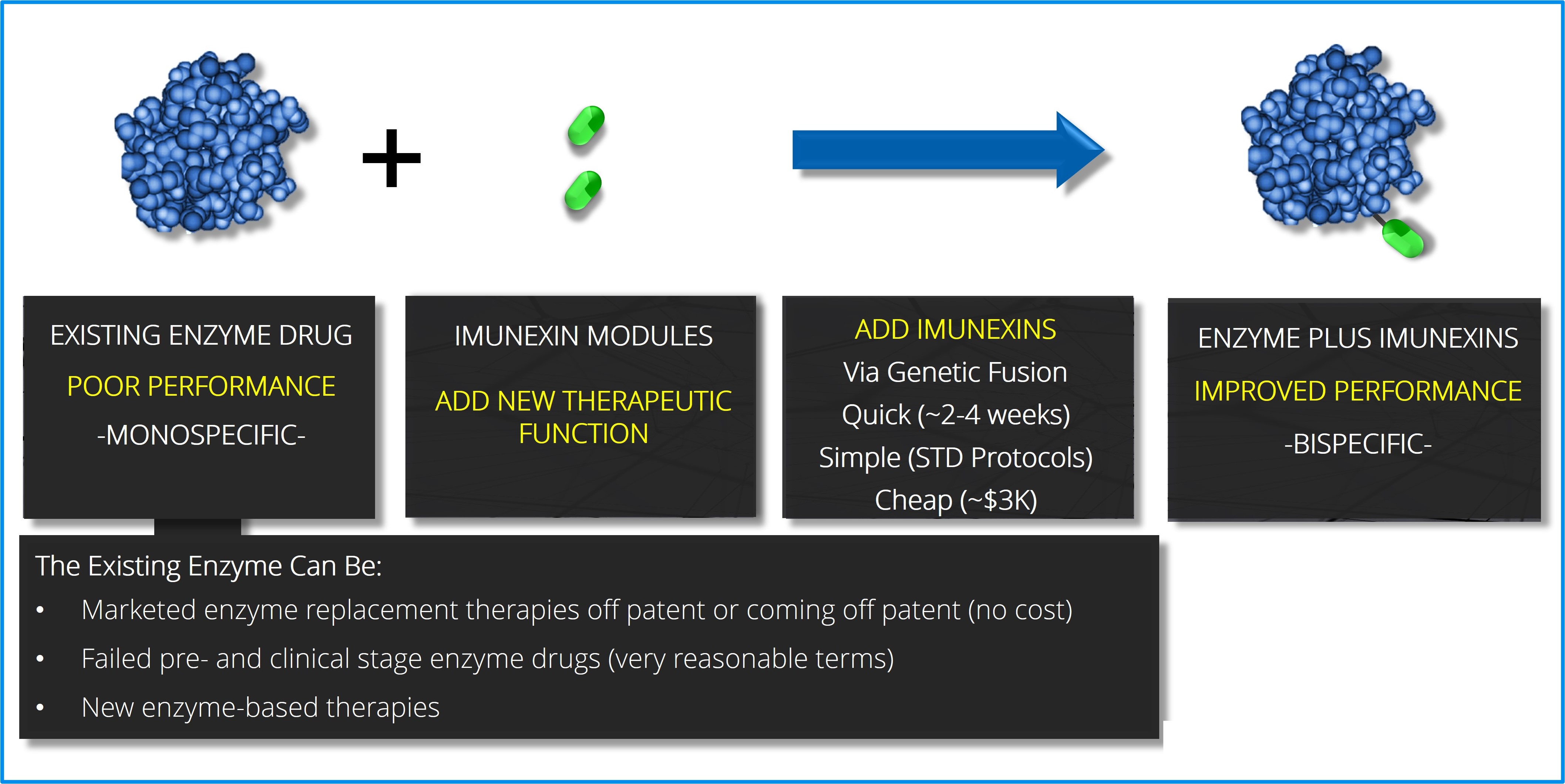
Diagram illustrating that imunexinsTM are added to existing enzymes or other proteins via a simple genetic fusion (cloning event) into the expression vector of the protein.
POTENTIAL APPLICATIONS FOR MULTISPECIFICs:
Multispecifics have the potential to become important therapeutics for a number of diseases. Some of the potential applications for multispecifics are outlined:
- Redirect and activate immune effector cells to specifically kill tumours.
- Bind to multiple targets and effect multiple pathogenic pathways.
- Bind to multiple sites on the one target cell or protein to increase specificity or induce synergistic induction.
- Target tumours that are heterogeneous.
- Increase the half-life.
- Add a targeting function to non-targeting proteins.
- Bind to transport proteins to allow proteins to cross biological barriers.

